Explain Why Three Different Solvent Mixtures Were Used
What color were your leaves to startwith. A solution of the sample containing a mixture of compounds is applied to the layer of adsorbent.

Mixtures And Solutions Learning Activity Package Create Your Own Comic Drawing Activities Chemistry Education
A non-polar solvent will dissolve non-polar substances.

. Which wavelengths of light were mostabsorbed in your leaves. For example if a sample consists of two components one more polar than the. Rank the solvents used in the experiment in order of increasing polarity.
To separate strongly basic components make a mixture of 10 NH 4 OH in methanol and then make a 1 to 10 mixture of this in dichlormethane. Table 282 records the elutropic series of one and two com-ponent solvents. Explain why solvent selection is one of the most critical and often time-consuming steps in TLC analysis.
Since the plates were kept the same in all the variations we can easily determine the differences in Rf values were due to the different polarities of the eluting substances. Technique used to separate a mixture into its component molecules. The TLC plates we were using were covered in a polar stationary substance thus the more polar a substance was the lower the Rf value.
Ethanolwater combinations are commonly used because ethanol has good dissolving ability for many organics but is also infinitely co-soluble with water. Solvent Extraction is Based on Which Law. We can also say that its a method of separating a compound which is soluble in an immiscible or a.
Not in the video but if you want to do chromatography with ink from a permanent. A polar solvent water will dissolve polar substances water soluble ink in the video below. Explain why solvent mixtures are often used rather than pure solvents to develop TLC plates.
Does the migration of each pigment relative to the migration of the solvent change. In the laboratory in order to separate effectively the analytes characteristics such as the polarity of the NaCl solution or the sodium chloride solution is being used. The support is usually a sheet of glass or metal foil.
Up to 256 cash back Why was itimportant to mark the TLC plate with pencil. You can use the same solvents in both your experiments and TLC. A solution has a solvent and at least one.
But alcohol is not the proper solvent because water and alcohol are highly miscible with each other. You may need touse both the TLC and spectrophotometricdata. Chromatography is one of the most known technique in separating components of a certain mixture.
Learn Exam Concepts on Embibe. If a different solvent were used for the. When no single solvent can be found that meets all of the criteria for crystallization it may be possible to use a mixed solvent.
How to solve reference front. Explain why solvent mixtures are often used rather than pure solvents to develop TLC plates. Why do you start with the least polar solventsolvent mixture and progress to increasing polar solventsolvent mixtures when eluting the ferrocene compounds from the column rather than starting with more polar solvent system and progressing to less polar.
Two different solvent mixtures of varying dilutions 20801 and 50501 were from CHEM 3321 at University of Colorado Boulder. A pair of solvents is chosen. The most common organic solvent used is ether.
A solvent that can be used for separating mixtures of strongly polar compounds is ethyl acetate. Using informationfrom the prelab lecture determine which pigments are present inyour various leaf samples. Different solvents will dissolve different substances.
From actual experimental results it has been established beyond any reasonable doubt that the mix-tures of two or three solvents of different polarity mostly offer distinct and much improved separation as compared to chemically homogeneous solvents. Solvent extraction is based on the distribution law. Different solvents will dissolve different substances.
3 powder used in the form of a thin layer about 025 mm thick on a supporting material. The mixture acetoneacetic acid 991 vv gave the best results for the qualitative and quantitative assay of the polyphenols present in. It is a method of separating compounds on the basis of their solubility in two different immiscible liquids like water and organic compound.
Distance the pigment traveled over the distance the solvent front traveled. Solvent extraction has always proved itself very helpful as a recovery method for many components. The second solvent solvent 2 should induce crystallization when added to a saturated solution of your compound in the primary solvent.
Solutions are the simplest mixture consisting of two substances that interact and have reasonably small particle sizes. The development solvent can be varied by using different solvents and 3 the polarity of the compounds in the spot this varies depending upon what compounds are in the spot. It was shown that water ethanol and n-octanol segregate in water-rich and octanol-rich mesoscopic.
However benzene and acetone can also be used. Using three different isotopic labeling conditions in small-angle neutron scattering SANS combined with small-angle high-res-olution X-ray scattering SWAXS could deliver unambiguous structural information 2. Chromatography is a technique used to separate the components of a mixture.
The three general types of mixtures in chemistry are solutions suspensions and colloids. -DCM-MeOH 401 301 201 101 51 -EtOAc-petroleum 31 21 1. All mixtures contain at least two different substances and can be liquids gases or solids.
Acetone containing extraction mixtures were superior to the ones containing methanol for extraction yield of total phenolic compounds which was especially pronounced for the groups of flavan-3-ols and conjugated forms of ellagic acid. Università degli Studi di Perugia. Why is ethanol a good solvent for recrystallization.
The mobile phase consists of a volatile organic solvent or mixture of solvents. The solvents that are alike in polarity for the stationary. Organic solvent of ether or acetone.
One in which the compound is soluble called the soluble solvent and one in which the compound is insoluble called the insoluble solvent.

Yummy Summer Drinks Mixtures Homogeneous Mixture

Learn About Solutions Science For Kids Teaching Science Science For Kids Chemistry Classroom

Solvent Mixture An Overview Sciencedirect Topics

Mixtures Vs Solutions Science Of Canning Science Worksheets Chemistry Worksheets Matter Science

Mixtures And Solutions Chemistry Lesson Plans Matter Science Middle School Chemistry

There Are Different Types Of Mixtures Solutions And Suspensions Chemistry Lessons Science Lessons Teaching Science

Separating Mixtures Science Educational School Posters Chemistry Classroom Gcse Science Teaching Chemistry
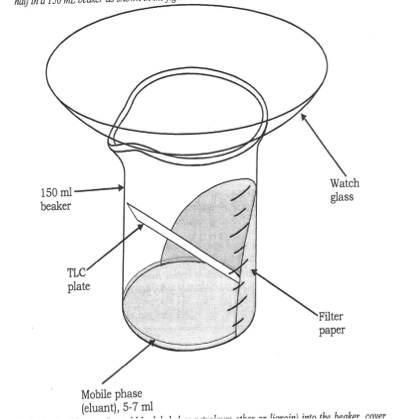
Thin Layer And Column Chromatography

Chem Is Fun Lab Separation Of A Mixture By Paper Chromatography Paper Chromatography Chemistry Lessons Matter Science

Chromatography Is A Sophisticated Method Of Separating Mixtures Of Two Or More Compounds The S Thin Layer Chromatography Science Chemistry Separating Mixtures

Solvent Mixture An Overview Sciencedirect Topics

Challenge Your Students And Their Understanding Of Physical And Chemical Changes With This Critical Think Teaching Chemistry Science Classroom Teaching Science

Solvent Mixture An Overview Sciencedirect Topics

Elements Compounds And Mixtures Odd One Out Worksheet Physical Science Lessons Middle School Science Compounds And Mixtures

Mixtures And Solutions Activities Notebook Worksheets Chemical Science Science Chart Mixture And Solution Activity

Mixtures Solutions Mixtures Vs Solutions Experiment Solutions And Mixtures Fourth Grade Science Solutions

Mixtures And Solutions In 2021 Matter Science Solutions And Mixtures Science Chemistry


Comments
Post a Comment